17-05-2024
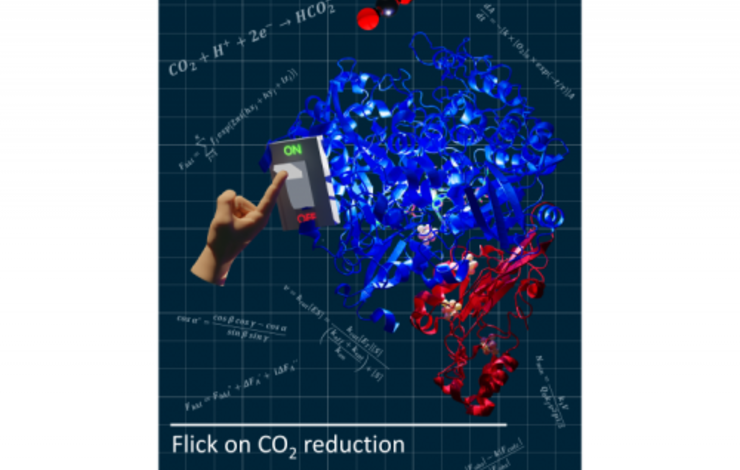
A new study, published in Nature Chemical Biology, by researchers from the Macromolecular Crystallography research lab at UCIBIO-NOVA, in collaboration with the group of Ines C Pereira at ITQB-NOVA, revealed how a formate dehydrogenase can withstand oxygen exposure. The findings reported in the paper “An allosteric redox switch involved in oxygen protection in a CO2 reductase” contribute to an enhanced comprehension of the enzyme's catalytic mechanism, providing insights into optimizing carbon dioxide reduction strategies, and therefore improve its future biotechnological applications.
European Union stands firm in its commitment to achieving carbon neutrality by 2050. To achieve this goal the reduction of greenhouse gases emissions must be complemented with the capture of carbon dioxide (CO2). Plants, soils, oceans, among other nature's elements responsible for CO2 removal from the atmosphere are not sufficient to achieve this objective, and thus it is urgent to find new catalysts that allow the transformation (reduction) of CO2 into other added-value products. Bacteria whose natural metabolism involves CO2 reduction are an option.
The same team has previously identified an enzyme capable of reducing carbon dioxide (CO2) from the atmosphere with high efficiency. The process is catalyzed by metal-dependent formate dehydrogenases (Fdhs), that transform CO2 into formate, a chemical fuel equivalent to hydrogen. Fdhs are enzymes found in one of the oldest and most energy efficient biological pathways.
However, while metal-dependent formate dehydrogenases are well-known for their efficiency to reduce CO2 with high efficiency and selectivity, they are usually very oxygen sensitive and must be purified and handled under strict anaerobic conditions.
In the work now published, researchers focused on clarifying the mechanism of CO2 reduction to formate by the highly efficient W-Fdh and unveiled an allosteric switch involved in the mechanism of oxygen protection of this enzyme.
The W-Fdh from the Sulfate-Reducing Bacterium Desulfovibrio vulgaris remains stable when purified and exposed to oxygen and can be stored for extended periods of time. However, in the laboratory, for the enzyme to reach maximum efficiently, a chemical pre-activation step was needed. This pre-activation step is reported in the literature since the 80’s, but no one understood, at the molecular level, how this could impact the activity of the enzyme.
The team has identified an allosteric mechanism triggered by forming or breaking a disulfide bridge, that either prevents enzyme inactivation when bacterial cells are exposed to oxygen or activates the enzyme under reducing and anaerobic conditions, to achieve the highest performance.
These results reveal new features of the catalytic mechanism, active site environment, and geometry of the W metal site, which are extremely valuable and may inspire new strategies for future biotechnological applications. This will enable to activate this enzyme in situ, when anaerobic conditions are achieved, by triggering the allosteric mechanism whenever the enzymatic reduction of CO2 to formic acid is required. The formate molecule has enormous potential, not only in directly powering batteries (DFAFCs – Direct Formic Acid Fuel Cells) for small electronic devices but also as a safer hydrogen storage molecule.
This work was recently published in Nature Chemical Biology:
An allosteric redox switch involved in oxygen protection in a CO2 reductase
Ana Rita Oliveira, Cristiano Mota, Guilherme Vilela-Alves, Rita Rebelo Manuel, Neide Pedrosa, Vincent Fourmond, Kateryna Klymanska, Christophe Léger, Bruno Guigliarelli, Maria João Romão & Inês A. Cardoso Pereira
Nature Chemical Biology, volume 20, pages111–119 (2024).